Search Results
Results for: 'organic compounds'
By: HWC, Views: 6073
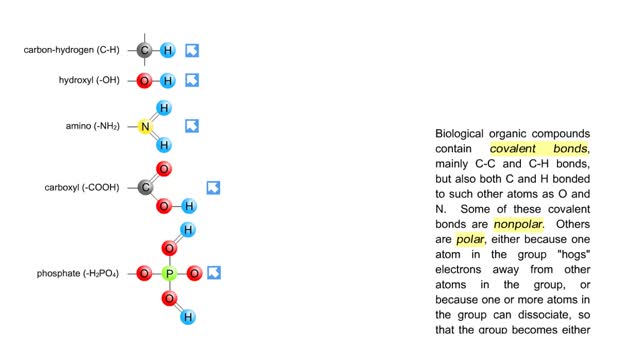
Biological organic compounds contain covalent bonds, mainly C-C and C-H bonds, but also both C and H bonded to such other atoms as O and N. Some of these covalent bonds are nonpolar. Others are polar, either because one atom in the group "hogs" electrons away from other atoms in the group, or...
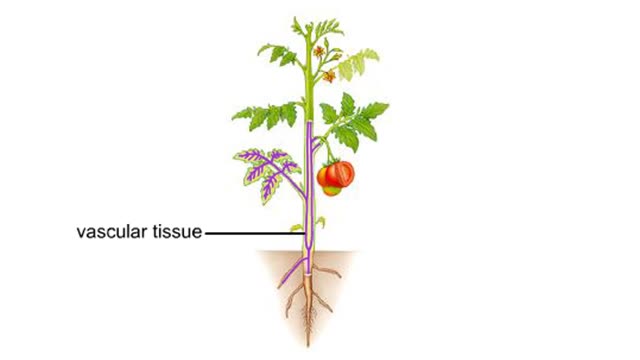
Vascular tissues in a corn stem and a buttercup root
By: HWC, Views: 1148
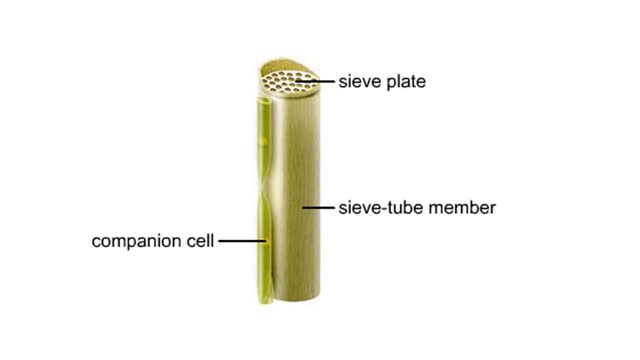
Vascular tissues in a corn stem and a buttercup root. The cells that make up each tissue. Xylem conducts water and dissolved ions. It also helps mechanically support a plant. The cells, called vessel members and tracheids, are dead at maturity. Their lignified walls interconnect and serve as p...
Miller's reaction chamber experiment Animation
By: HWC, Views: 272
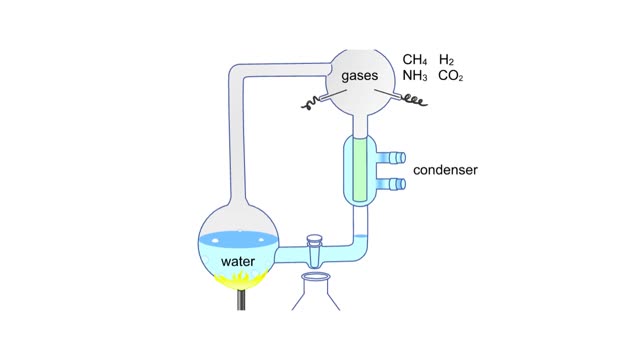
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. ...
Non-polar compounds - insolubility
By: HWC, Views: 6686
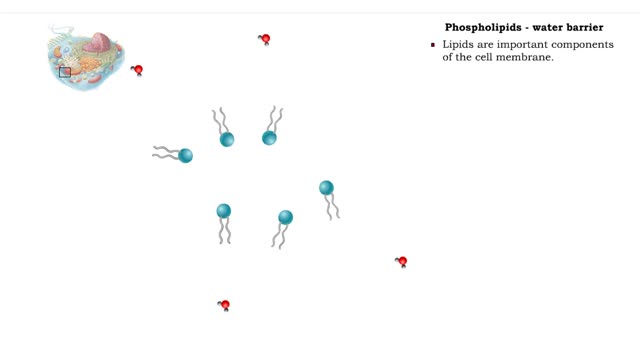
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
By: HWC, Views: 1226
The bulk of the plant body is comprised of ground tissue. Vascular tissue threads through the ground tissue. It distributes water, solutes, and organic substances through the plant body. Dermal tissue covers and protects the surfaces of the root and shoot systems.
By: HWC, Views: 844
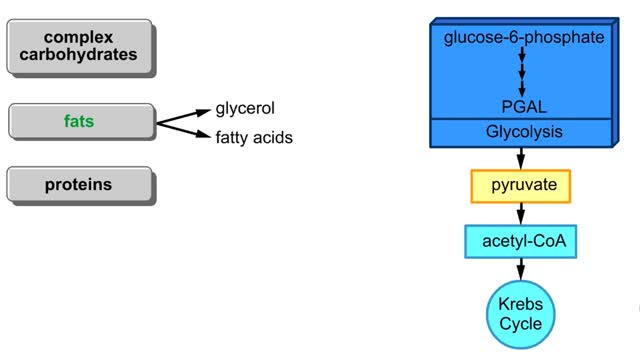
Points at which organic compounds enter the reaction stages of aerobic respiration. Complex carbohydrates are broken down into simple sugars, such as glucose. They become the substrates for glycolysis. If your body doesn't need to burn glucose for energy, glucose-6-phosphate can be co...
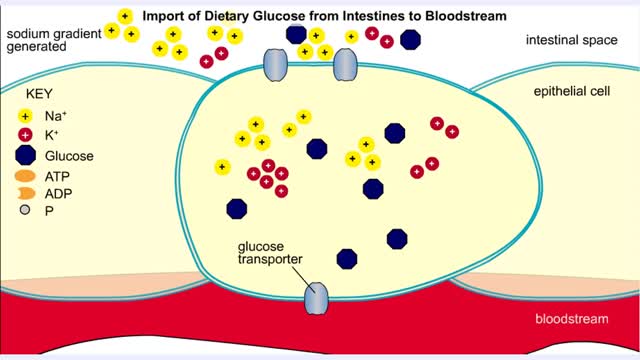
Import of Dietary Glucose from Intestines to Bloodstream
By: HWC, Views: 6107
• Membranes have hydrophobic interiors. which resist the passage of hydrophilic compounds and ions. • However. transporter membrane proteins facilitate the passage of these molecules. • Passive transporters accelerate diffusion of molecules towards equilibrium (decrease a concentrat...
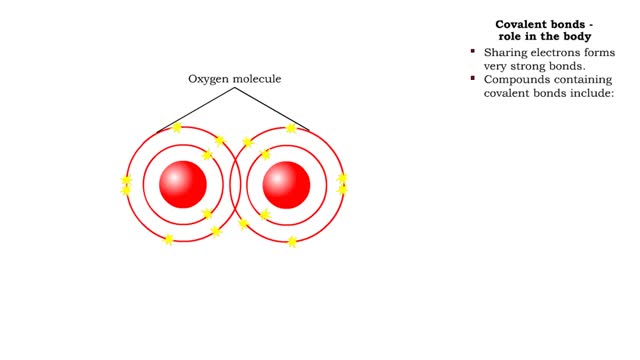
Covalent bonds - role in the body
By: HWC, Views: 6648
A covalent bond is formed when atoms share one or more pairs of electrons. This is opposed to an ionic bond, where electrons are actually transferred from one atom to another. Formation • Atoms fill up the outer orbital by sharing electrons. • Two oxygen atoms sharing electrons form on...
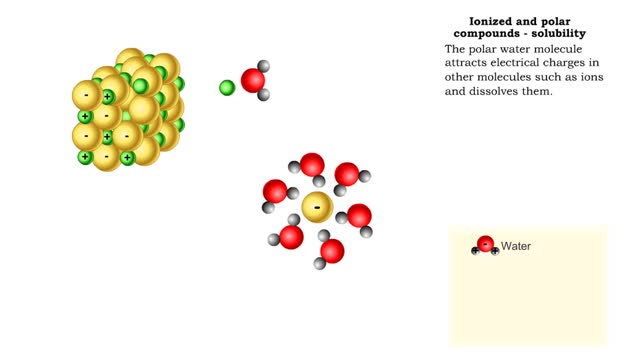
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 6822
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Advertisement